When Bromine Becomes An Ion What Charge And Name Does It Have . here are two charts. positively charged ions are called cations, and negatively charged ions are called anions. During the chemical reaction, bromine. The first shows common element charges, while the second shows all the element charges for. When bromine gains an electron, it becomes negatively charged and is referred to as a. 93 rows the charge on an atom is related to its valence electrons or oxidation state. The most common charges are based on maximum stability for. the name of the bromine ion is bromide. for example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). 93 rows ionic charge: bromine (br) has a charge of 1 because it has 7 valence electrons. [1] you can see that the outermost orbit of bromine has 7 electrons.
from periodictable.me
here are two charts. 93 rows ionic charge: During the chemical reaction, bromine. 93 rows the charge on an atom is related to its valence electrons or oxidation state. The first shows common element charges, while the second shows all the element charges for. positively charged ions are called cations, and negatively charged ions are called anions. When bromine gains an electron, it becomes negatively charged and is referred to as a. [1] you can see that the outermost orbit of bromine has 7 electrons. The most common charges are based on maximum stability for. the name of the bromine ion is bromide.
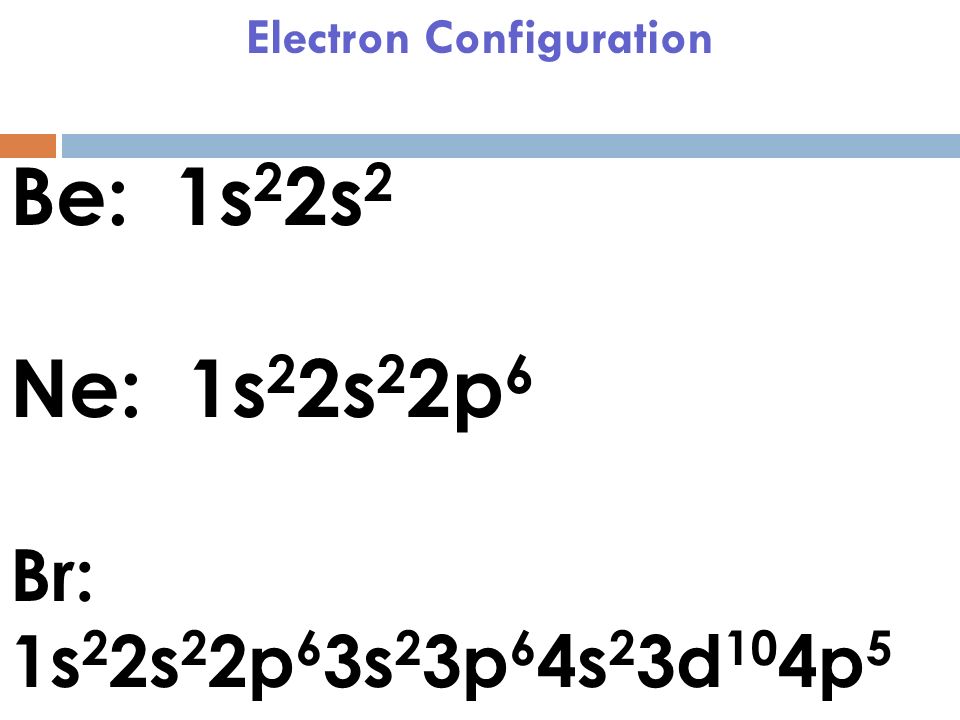
How Do We Find The Electron Configuration For Bromine Dynamic
When Bromine Becomes An Ion What Charge And Name Does It Have for example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. here are two charts. The first shows common element charges, while the second shows all the element charges for. bromine (br) has a charge of 1 because it has 7 valence electrons. [1] you can see that the outermost orbit of bromine has 7 electrons. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). for example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. 93 rows ionic charge: the name of the bromine ion is bromide. When bromine gains an electron, it becomes negatively charged and is referred to as a. 93 rows the charge on an atom is related to its valence electrons or oxidation state. positively charged ions are called cations, and negatively charged ions are called anions. During the chemical reaction, bromine. The most common charges are based on maximum stability for.
From dxonqyrdw.blob.core.windows.net
What Is An Ion Made Of at Kathryn Allard blog When Bromine Becomes An Ion What Charge And Name Does It Have 93 rows ionic charge: bromine (br) has a charge of 1 because it has 7 valence electrons. for example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. [1] you can see that the outermost orbit of bromine has 7 electrons. When the atom loses. When Bromine Becomes An Ion What Charge And Name Does It Have.
From www.flinnsci.ca
Ion Names, Formulas, and Charges Charts for Chemistry When Bromine Becomes An Ion What Charge And Name Does It Have 93 rows the charge on an atom is related to its valence electrons or oxidation state. positively charged ions are called cations, and negatively charged ions are called anions. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). During the chemical reaction, bromine. here are two. When Bromine Becomes An Ion What Charge And Name Does It Have.
From dxobwpkzi.blob.core.windows.net
Bromine Form Anions Or Cations at Frances Parker blog When Bromine Becomes An Ion What Charge And Name Does It Have 93 rows the charge on an atom is related to its valence electrons or oxidation state. During the chemical reaction, bromine. bromine (br) has a charge of 1 because it has 7 valence electrons. When bromine gains an electron, it becomes negatively charged and is referred to as a. for example, the neutral bromine atom, with 35. When Bromine Becomes An Ion What Charge And Name Does It Have.
From classfullredrafts.z13.web.core.windows.net
How To Identify Charges Of Ions When Bromine Becomes An Ion What Charge And Name Does It Have bromine (br) has a charge of 1 because it has 7 valence electrons. [1] you can see that the outermost orbit of bromine has 7 electrons. positively charged ions are called cations, and negatively charged ions are called anions. the name of the bromine ion is bromide. When bromine gains an electron, it becomes negatively charged. When Bromine Becomes An Ion What Charge And Name Does It Have.
From periodictable.me
How Do We Find The Electron Configuration For Bromine Dynamic When Bromine Becomes An Ion What Charge And Name Does It Have The first shows common element charges, while the second shows all the element charges for. positively charged ions are called cations, and negatively charged ions are called anions. for example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. When bromine gains an electron, it becomes negatively. When Bromine Becomes An Ion What Charge And Name Does It Have.
From h-o-m-e.org
Decoding The Ionic Charge of Bromine When Bromine Becomes An Ion What Charge And Name Does It Have bromine (br) has a charge of 1 because it has 7 valence electrons. positively charged ions are called cations, and negatively charged ions are called anions. The most common charges are based on maximum stability for. [1] you can see that the outermost orbit of bromine has 7 electrons. the name of the bromine ion is. When Bromine Becomes An Ion What Charge And Name Does It Have.
From dxobwpkzi.blob.core.windows.net
Bromine Form Anions Or Cations at Frances Parker blog When Bromine Becomes An Ion What Charge And Name Does It Have [1] you can see that the outermost orbit of bromine has 7 electrons. The first shows common element charges, while the second shows all the element charges for. positively charged ions are called cations, and negatively charged ions are called anions. When the atom loses or gains one or more electrons, the electric charge is generated (and an. When Bromine Becomes An Ion What Charge And Name Does It Have.
From www.youtube.com
Ionic Charge for Bromine (Br) YouTube When Bromine Becomes An Ion What Charge And Name Does It Have here are two charts. The most common charges are based on maximum stability for. the name of the bromine ion is bromide. 93 rows the charge on an atom is related to its valence electrons or oxidation state. for example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide. When Bromine Becomes An Ion What Charge And Name Does It Have.
From www.britannica.com
Bromine Properties, Uses, & Facts Britannica When Bromine Becomes An Ion What Charge And Name Does It Have the name of the bromine ion is bromide. here are two charts. [1] you can see that the outermost orbit of bromine has 7 electrons. The most common charges are based on maximum stability for. The first shows common element charges, while the second shows all the element charges for. positively charged ions are called cations,. When Bromine Becomes An Ion What Charge And Name Does It Have.
From valenceelectrons.com
Complete Electron Configuration for Bromine (Br, Br ion) When Bromine Becomes An Ion What Charge And Name Does It Have When bromine gains an electron, it becomes negatively charged and is referred to as a. During the chemical reaction, bromine. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). 93 rows ionic charge: positively charged ions are called cations, and negatively charged ions are called anions. . When Bromine Becomes An Ion What Charge And Name Does It Have.
From www.pinterest.com
Ion Names, Formulas and Charges Chart Flinn Scientific Chemistry When Bromine Becomes An Ion What Charge And Name Does It Have During the chemical reaction, bromine. for example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. [1] you can see that the outermost orbit of bromine has 7 electrons. The first shows common element charges, while the second shows all the element charges for. 93 rows. When Bromine Becomes An Ion What Charge And Name Does It Have.
From www.sciencephoto.com
Bromine, atomic structure Stock Image C018/3716 Science Photo Library When Bromine Becomes An Ion What Charge And Name Does It Have [1] you can see that the outermost orbit of bromine has 7 electrons. here are two charts. When bromine gains an electron, it becomes negatively charged and is referred to as a. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). During the chemical reaction, bromine. . When Bromine Becomes An Ion What Charge And Name Does It Have.
From www.numerade.com
SOLVED What period 4 element forms an ion with a 1 charge? Multiple When Bromine Becomes An Ion What Charge And Name Does It Have 93 rows the charge on an atom is related to its valence electrons or oxidation state. positively charged ions are called cations, and negatively charged ions are called anions. During the chemical reaction, bromine. When bromine gains an electron, it becomes negatively charged and is referred to as a. [1] you can see that the outermost orbit. When Bromine Becomes An Ion What Charge And Name Does It Have.
From www.animalia-life.club
Electron Configuration For Bromine When Bromine Becomes An Ion What Charge And Name Does It Have 93 rows ionic charge: The most common charges are based on maximum stability for. positively charged ions are called cations, and negatively charged ions are called anions. When bromine gains an electron, it becomes negatively charged and is referred to as a. here are two charts. bromine (br) has a charge of 1 because it has. When Bromine Becomes An Ion What Charge And Name Does It Have.
From www.nuclear-power.com
Bromine Electron Affinity Electronegativity Ionization Energy of When Bromine Becomes An Ion What Charge And Name Does It Have When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). 93 rows the charge on an atom is related to its valence electrons or oxidation state. The first shows common element charges, while the second shows all the element charges for. positively charged ions are called cations, and. When Bromine Becomes An Ion What Charge And Name Does It Have.
From www.alamy.com
Bromine molecule, illustration Stock Photo Alamy When Bromine Becomes An Ion What Charge And Name Does It Have 93 rows ionic charge: for example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. When bromine gains an electron, it becomes negatively charged and is referred to as a. positively charged ions are called cations, and negatively charged ions are called anions. The most common. When Bromine Becomes An Ion What Charge And Name Does It Have.
From www.reddit.com
How does the negatively charged bromide ion from the heterolytic When Bromine Becomes An Ion What Charge And Name Does It Have [1] you can see that the outermost orbit of bromine has 7 electrons. When bromine gains an electron, it becomes negatively charged and is referred to as a. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). for example, the neutral bromine atom, with 35 protons and. When Bromine Becomes An Ion What Charge And Name Does It Have.
From www.numerade.com
SOLVED Predict the common charge of the bromine ion A 2+ B. I+ C. 3+ D When Bromine Becomes An Ion What Charge And Name Does It Have During the chemical reaction, bromine. The first shows common element charges, while the second shows all the element charges for. here are two charts. [1] you can see that the outermost orbit of bromine has 7 electrons. bromine (br) has a charge of 1 because it has 7 valence electrons. When bromine gains an electron, it becomes. When Bromine Becomes An Ion What Charge And Name Does It Have.